Microbiome

“Without bacteria, viruses, fungi, and other microorganisms, there wouldn’t be life on earth, and we wouldn’t be here,” says Lita Proctor. “Every kind of living thing is dependent on communities of microbes we call microbiomes, and that includes humans.”
Proctor is director of the Human Microbiome Project, an eight-year research mission of the National Institutes of Health to explore the role of the microbiome in human health and disease. Here’s some of what we know so far...and what we still need to find out.
So much of our human history of exposure to bacteria and viruses has been through infectious diseases and plagues,” says Lita Proctor.
“It’s hard to wrap our heads around the idea that microbes also play a major role in supporting our health.”
They break down undigested food, make vitamins, prime our immune system, secrete neurotransmitters that allow nerve cells to communicate with each other, help defend us against invading bugs, and more.
“But there’s still so much that we don’t know,” says Elisabeth Bik, of Stanford University, who publishes microbiomedigest.com, a website that updates investigators on new research.
Some of what we know
Your microbiome begins at birth. You’re colonized with your mother’s vaginal microbiome as you pass through the birth canal. Babies born by caesarean section, who miss that journey, are colonized instead largely with microbes from the hands of their mothers and the nurses, doctors, or others who hold them.
That may matter later. Children born by C-section, for example, are nearly twice as likely to have asthma.1
Rob Knight, a leading microbiome expert, and his wife, Amanda, knew the importance of that first microbiome when their daughter was born by emergency C-section.
“We took matters into our own hands,” the University of California, San Diego, scientist wrote in his recent book “Follow Your Gut.”
Using sterile cotton swabs, they took samples of Amanda’s vaginal microbiome, “which we then transferred to various parts of our newborn: the skin, the ears, the mouth—all the places microbes would have ended up naturally had she passed through the birth canal,” wrote Knight. “Our baby needed those microbes.”
You have many microbiome communities in and on your body. The microbiome in the large intestine is by far the largest and most complex. “The average adult carries three to five pounds of microbes there,” notes Proctor. Our fingers, hands, ears, navels, toes, mouth, and other nooks and crannies each have their own unique community of bacteria, viruses, and yeasts.
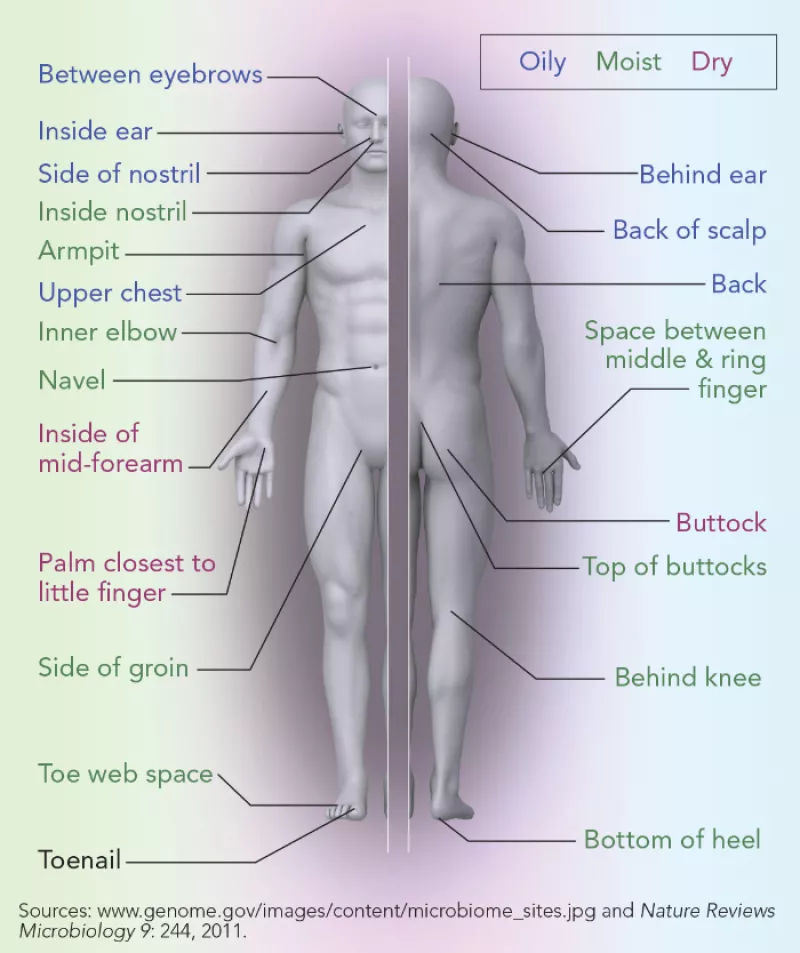
Your skin and other sites on your body are home to distinct communities of bacteria, fungi, and viruses.
The environment affects your microbiome. If you live with other people, your microbiome bears some resemblance to theirs, even if they’re not blood relatives. Have a dog, cat, or other pet? You’ve picked up some of its microbiome, too.
No two people have the same microbiome. As a group, humans have similar microbiomes. “But if you drill down deeper, you’ll see differences in the composition of the microbiomes between one person and another,” says Proctor.
For example, University of Colorado researchers could link nine people to their computer mice by matching the bacteria left on the mice to the microbiomes on the owners’ fingers.2
But that doesn’t mean that different microbiomes function differently.
“Microbes are metabolically incredibly versatile,” Proctor explains. You can have two microbiomes that look very different but function in the same way. “That’s because microbiomes are communities of microbes, not individuals acting independently.”
There is no “best” microbiome. “There is no one healthy microbiome,” notes Bik. “There’s a huge variety of microbiomes among healthy people.”
What’s more, a healthier gut microbiome has many families of microbes. But for the vaginal microbiome, less diversity seems to be healthier.
“This drives researchers crazy,” says Proctor, “because they want clear rules of the road.”
The best food for your gut microbiome is fiber. “Eating dietary fiber is associated with a very different microbiome, one that’s richer, with a higher number of bacterial species compared with eating a low-fiber diet,” says Bik.
“But you can’t get the fiber from a drink or a pill,” notes Proctor. “You have to present your microbes with a diverse array of fibers from a lot of different plant materials, such as beans and green vegetables.”
Think of yourself as a zookeeper for the community of little creatures inside you, says Bik. “Eating fiber is good not just for us: It also keeps them happy.”

Fresh fruits and vegetables are a much richer source of healthy microbes than processed foods.
Antibiotics disrupt your gut microbiome. “When we take antibiotics, we lose good and bad bacteria and some of the diversity in our gut,” says Bik. “Some of the microbes will bounce back, but some probably won’t.”
Your gut microbiome changes as you age. It gets “noisier,” says Proctor. “We find less diversity—fewer families of microbes—in the microbiomes of people 65 and older, and there’s a lot more variability between older people than between younger folks.”
That could be the result of repeated antibiotic use, says Bik. “Or maybe they’re eating softer, less fibrous food or spending less time outdoors exposed to microbes.”
Bottom line: Don’t cut back on fiber-rich plant foods as you get older, says Proctor.
Some of what we don't know
How does the microbiome affect weight? During the exhilarating first years of microbiome research, scientists reported differences in the makeup of gut bacteria between lean and overweight people.
Researchers even succeeded in fattening lean mice by transferring microbes from overweight people into their colons. That raised the hope that obesity could one day be treated with microbes.
But this summer, two University of Michigan researchers pooled the results of 10 studies on nearly 2,800 people of varying weights.3
Their finding: knowing which microbes are in a person’s gut microbiome isn’t very good at predicting whether he or she is normal weight or obese. In other words, they could find no clear “lean” or “obese” gut microbiome.
“It is possible that each individual has his or her own signatures of obesity,” the researchers speculated.
That makes sense to Lita Proctor.
“You can have different bacteria performing the same functions, so knowing only their identity may not tell you what you need to know,” she notes.
It’s what those microbes do, not who they are, that may matter.
“Researchers are now measuring the activity of these microbes—what proteins and other metabolites they’re producing as a community, for example—to get closer to knowing how a particular microbiome affects our health,” says Proctor.
Can the microbiome prevent or treat disease? Scientists are hot on the trail, but have yet to figure out how—or even if—it does. Exception: treating Clostridium difficile infections. (See “Poop Power?” below.)
Some examples:
Diabetes. In 2010, Dutch researchers reduced insulin resistance—which often leads to type 2 diabetes—in nine men by transplanting into their intestines solutions containing the feces of men who were not insulin resistant.4
Can fecal transplants actually prevent—or even help reverse—diabetes? Several studies are looking.
Depression. In an Irish study, the gut microbiomes of 34 people with serious depression had fewer and less diverse microbes than the gut microbiomes of 33 similar depression-free people.5
Interestingly, rats that got transplants of fecal microbes from the people with depression were more likely to show signs of anxiety and less interest in appealing food than rats that got transplants from people without depression.
Autism. Small pilot studies find that the gut microbiomes of children with autism are different from other children's gut microbiomes.6 But that doesn't mean that the microbiomes caused the autism. Autistic children often have diarrhea or constipation or abnormal eating habits, which could alter their microbiomes.
But when researchers at the California Institute of Technology transferred gut microbes from autistic children into mice, the animals started exhibiting repetitive behaviors and had trouble interacting with others, two characteristics of people with autism. The study hasn't yet been published.
Colorectal cancer. People with colorectal cancer have different gut microbiomes than others, but that could be either a cause or a result of the cancer.7
The evidence for the microbiome as culprit isn't clear. After being exposed to a carcinogen, mice that got fecal transplants from other mice with colorectal cancer developed more and larger tumors than mice that got fecal transplants from healthy mice.8
But when researchers exposed mice to a carcinogen after giving them fecal transplants from humans with colon cancer, the mice had no more tumors than mice that got transplants from healthy people.7
Heart disease. Red-meat eaters have bacteria in their gut microbiomes that convert the carnitine in meat into what eventually becomes TMAO, a compound that speeds up artery clogging.9 That may help explain why people who eat red meat have a higher risk of heart disease.
Vegetarians have less of that kind of bacteria, and they don't produce much TMAO when given a carnitine supplement.
To play it safe, avoid supplements that contain carnitine, unless they've been prescribed.
Vaginosis. Bacterial vaginosis—the most common vaginal infection in women up to age 44—occurs when the normal balance of bacteria in the vagina is disrupted.
But vaginal microbiomes in healthy women can differ among ethnic groups, and within the same group depending on age, birth control methods, and sexual practices.10
For example, Lactobacilli dominate most vaginal microbiomes, but a microbiome that's short on Lactobacilli isn't necessarily unhealthy.
So far, two studies—in Brazil and Benin, Africa—suggest that an oral probiotic with Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14, along with an antibiotic, helped clear up vaginosis better than an antibiotic alone.11,12 But more studies are needed.
Asthma. Children who are exposed to a host of microbes in the environment while growing up—if they live on a farm, for example—are less likely to have asthma. But studies looking at whether more diverse gut microbiomes are linked to a lower risk are inconsistent.13 Nor have studies consistently implicated a specific group of microbes as risky.
And in four trials, children aged five months and younger who were given probiotics for 3 to 9 months were just as likely to develop asthma or wheezing as those given a placebo.14
Ulcerative colitis. The gut microbiomes of people with chronic inflammation and sores in the lining of their colon have lower diversity and a different makeup of microbes than the microbiomes of healthy people.15 But it's not clear whether that's a cause or a result of the disease.
Canadian researchers gave 38 patients weekly fecal-transplant enemas for six weeks. By the end of the study, 9 were in remission, compared with just 2 of 37 who got placebo enemas.16
1Thorax 64: 107, 2009.
2PNAS 107: 6477, 2010.
3Mbio 7: e01018-16, 2016.
4Gastroenterology 143: 913, 2012.
5J. Psychiatr. Res. 82: 109, 2016.
6Microb. Ecol. Health Dis. 26: 26914, 2015.
7Microbiome 2: 20, 2014.
8Mbio 4: e00692-13, 2013.
9N. Engl. J. Med. 368: 1575, 2013.
10Clin. Lab. Med. 34: 747, 2014.
11Can. J. Microbiol. 55: 133, 2009.
12Eur. J. Obstet. Gyn. Reprod. Biol. 168: 75, 2013.
13Yale J. Biol. Med. 89: 309, 2016.
14Pediatrics 132: e666, 2013.
15Transplant Proc. 48: 402, 2016.
16Gastroenterology 149: 102, 2015.
Poop power?

The most dramatic use of the gut microbiome so far has been the cure of difficult-to-treat, sometimes life-threatening Clostridium difficile infections using fecal transplants.1 Those are transfers of a solution containing a healthy donor’s stool to another person, either through a tube down the throat or an enema-like injection.
But the Food and Drug Administration has said that it will allow fecal transplants only to treat C. diff, and only when all other treatment options have been exhausted. Why? Concerns about safety and effectiveness.
Case in point: a 32-year-old near-normal-weight woman became obese after a fecal transplant to treat her C. diff infection.2 “This patient’s weight gain was dramatic and disturbing,” her physician told Scientific American. The donor was her 16-year-old daughter, who also became obese.
Coincidence? Researchers don’t know. But that hasn’t stopped websites like thepowerofpoop.com from helping people who want to do their own fecal transplants at home.
1World J. Gastroenterol. 21: 5359, 2015.
2Open Forum Infect. Dis. 2: ofv004, 2015.
Tags
Topics

