Letter in opposition to CBD Bill, H.R. 841
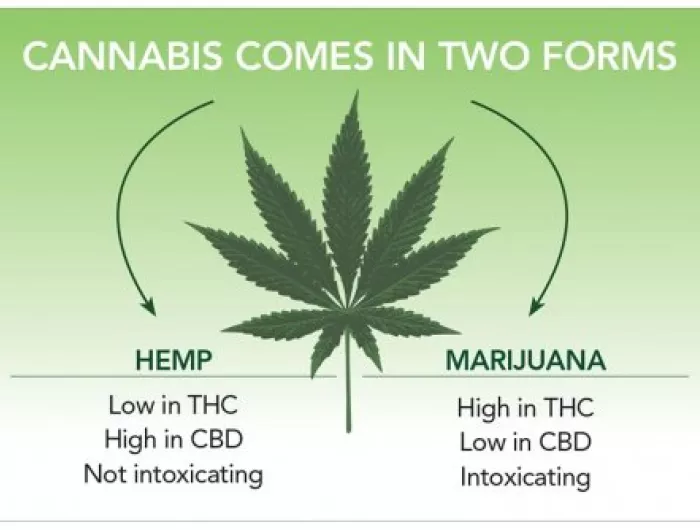
The Food and Drug Administration, and not Congress, should be the body that determines when and under what conditions cannabidiol, or CBD, and other hemp-derived ingredients are approved for use in dietary supplements and foods, according to the Center for Science in the Public Interest, Consumer Federation of America, and Consumer Reports. The three organizations are urging Members of Congress to oppose H.R. 841, which would force regulators at the FDA to immediately and categorically legalize CBD in supplements and foods, even though the FDA is currently still evaluating the safety implications of such a move.

